In this investigation, students classify chemical reactions as exothermic or endothermic. Next, students explore the relationship between an observed change in temperature and the classification of a change as chemical or physical.
Students will explore energy changes during chemical reactions, heat of reaction (ΔH), and the connection between energy changes and chemical changes.
One class period, approximately 45–50 minutes.
After students explore one example of an endothermic change and one example of an exothermic change, they are then asked to explore the connection between energy changes and chemical reactions. To do this, students may need some guidance to arrive at the idea that temperature changes may also accompany dissolving.
Students will have an easier time devising a fair test if they are well versed in the definitions of physical changes and chemical changes. Students should propose an experiment to you before they test their hypothesis. To observe a temperature change during a physical change, students should devise a procedure such as:
This investigation introduces the concepts of enthalpy (heat) of ΔH in the context of exothermic and endothermic reactions. To give students a deeper grounding in the basics and reinforce basic concepts covered previously, you may wish to review the mechanics of chemical changes, how to write balanced chemical equations, and the law of conservation of energy.
This investigation could be incorporated into a unit on chemical changes or thermochemistry.
In this activity, you will explore the energy changes that accompany chemical reactions. To understand the energy implications of chemical reactions, it’s important to keep in mind two key ideas:
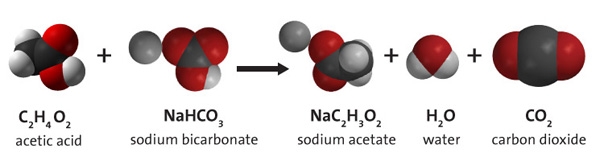
To understand this, consider the chemical reaction between vinegar (also known as acetic acid to chemists) and baking soda (known as sodium bicarbonate). Before the atoms of acetic acid and sodium bicarbonate can be rearranged to form the products, the bonds between the atoms in those molecules must be broken, and because the atoms are attracted to one another, it takes energy to pull them apart.

Then, when the products are formed (sodium acetate, water, and carbon dioxide) energy is released because atoms that have an attraction for one another are brought back together. Not every bond between atoms in the reactants is necessarily broken during a chemical reaction, but some bonds are.
By comparing the energy used when bonds in the reactants are broken with the energy released when bonds in the products are formed, you can determine whether a chemical reaction releases energy or absorbs energy overall.
Chemical reactions that release energy are called exothermic. In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the bonds in the reactants. Chemical reactions that absorb (or use) energy are called endothermic. In endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are formed in the products. If a chemical reaction absorbs as much energy as it releases, it is called isothermic—there is no net energy change.
But because we can’t observe bonds breaking or being formed, how can we distinguish between exothermic and endothermic chemical reactions?
There are two methods for distinguishing between exothermic and endothermic reactions.
When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. When energy is absorbed in an endothermic reaction, the temperature decreases. You can monitor changes in temperature by placing a thermometer in the reaction mixture.
To classify the net energy output or input of chemical reactions, you can calculate something called the enthalpy change (ΔH) or heat of reaction, which compares the energy of the reactants with the energy of the products.
Enthalpy is a measure of internal energy. So, when you calculate the difference between the enthalpy of the products and the enthalpy of the reactants, you find the enthalpy change (ΔH), which can be represented mathematically as:
ΔH = energy used in reactant bond breaking + energy released in product bond making
Wait, how can you find a difference by adding? The enthalpy values are added in the equation above because, by definition, energy used in reactant bond breaking is always positive (+) and energy released in product bond making is always negative (−).
If ΔH is negative (−) then the chemical reaction is exothermic, because more energy is released when the products are formed than energy is used to break up the reactants. If ΔH is positive (+) then the chemical reaction is endothermic, because less energy is released when the products are formed than the energy is used to break up the reactants.
You can also use energy level diagrams to visualize the energy change during a chemical reaction as a result of the energies used and released according to the above equation for ΔH. To understand these diagrams, compare the energy level of the reactants on the lefthand side with that of the products on the right-hand side.